-
 Latex-Free Powder-Free Nitrile Gloves
Latex-Free Powder-Free Nitrile Gloves -
 Comfortable Disposable Nitrile Gloves
Comfortable Disposable Nitrile Gloves -
 Powder Free Disposable Nitrile Glove
Powder Free Disposable Nitrile Glove -
 Disposable Powder Free Black Nitrile Glove
Disposable Powder Free Black Nitrile Glove -
 Powder Free Black Disposable Examination Nitrile Glove
Powder Free Black Disposable Examination Nitrile Glove -
 Nitrile Gloves Disposable Powder-Free Glove
Nitrile Gloves Disposable Powder-Free Glove -
 Disposable Powder Free Exam Nitrile Glove
Disposable Powder Free Exam Nitrile Glove -
 Medical Exam Disposable Powder Free Nitrile Glove
Medical Exam Disposable Powder Free Nitrile Glove -
 Disposable Powder Free Nitrile Glove
Disposable Powder Free Nitrile Glove
-
 Bluetooth Portable Fingertip Pulse Oximeter
Bluetooth Portable Fingertip Pulse Oximeter -
 Bluetooth Fingertip Pulse Oximeter Digital
Bluetooth Fingertip Pulse Oximeter Digital -
 Medical Grade Fingertip Pulse Oximeter
Medical Grade Fingertip Pulse Oximeter -
 Wireless Fingertip Pulse Oximeter
Wireless Fingertip Pulse Oximeter -
 SPO2 Fingertip Pulse Oximeter Blood Oxygen Monitor
SPO2 Fingertip Pulse Oximeter Blood Oxygen Monitor -
 Portable Pulse Oximeter for the Fingertip
Portable Pulse Oximeter for the Fingertip -
 SpO2 Digital Finger Fingertip Pulse Oximeter
SpO2 Digital Finger Fingertip Pulse Oximeter -
 Pulse Oximeter Handheld Digital Fingertip Pulse Oximeter
Pulse Oximeter Handheld Digital Fingertip Pulse Oximeter -
 Fingertip Digital Pulse Oximeter
Fingertip Digital Pulse Oximeter -
 Fingertip Pulse Oximeter Bluetooth Digital
Fingertip Pulse Oximeter Bluetooth Digital -
 Blood Oxygen Monitor SPO2 Fingertip Pulse Oximeter
Blood Oxygen Monitor SPO2 Fingertip Pulse Oximeter -
 Portable LED Display SPO2 Fingertip Pulse Oximeter
Portable LED Display SPO2 Fingertip Pulse Oximeter
KINGSTAR INC
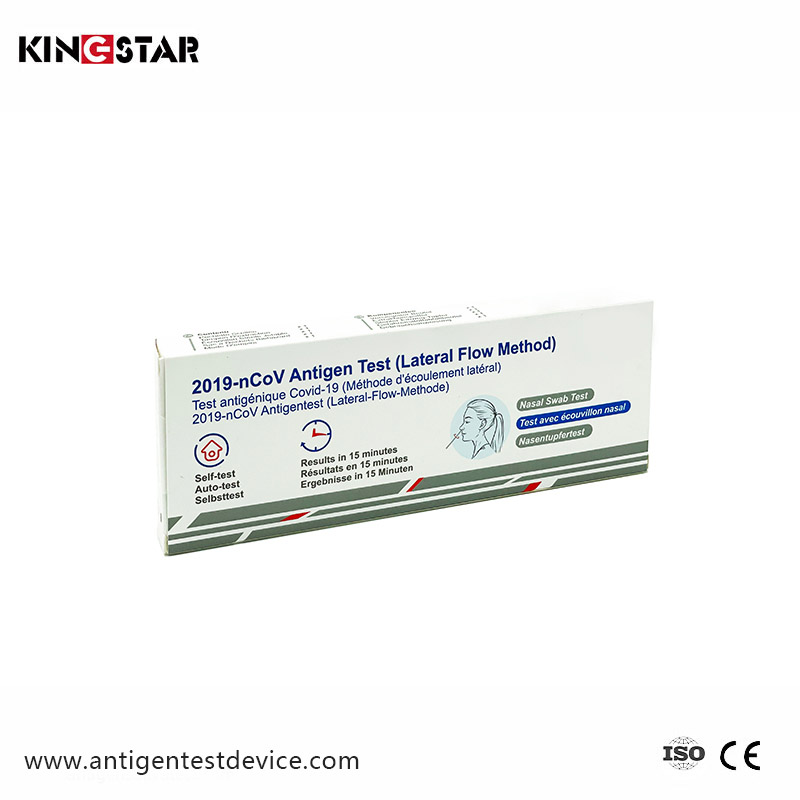
As we all know, in late 2019, the first case of a never-before-seen virus pneumonia, later named novel coronavirus pneumonia, was reported in Wuhan, China.
From Jan 21st, 2020, COVID-19 was confirmed as a type of human-to-human transmission virus and spread to more people.
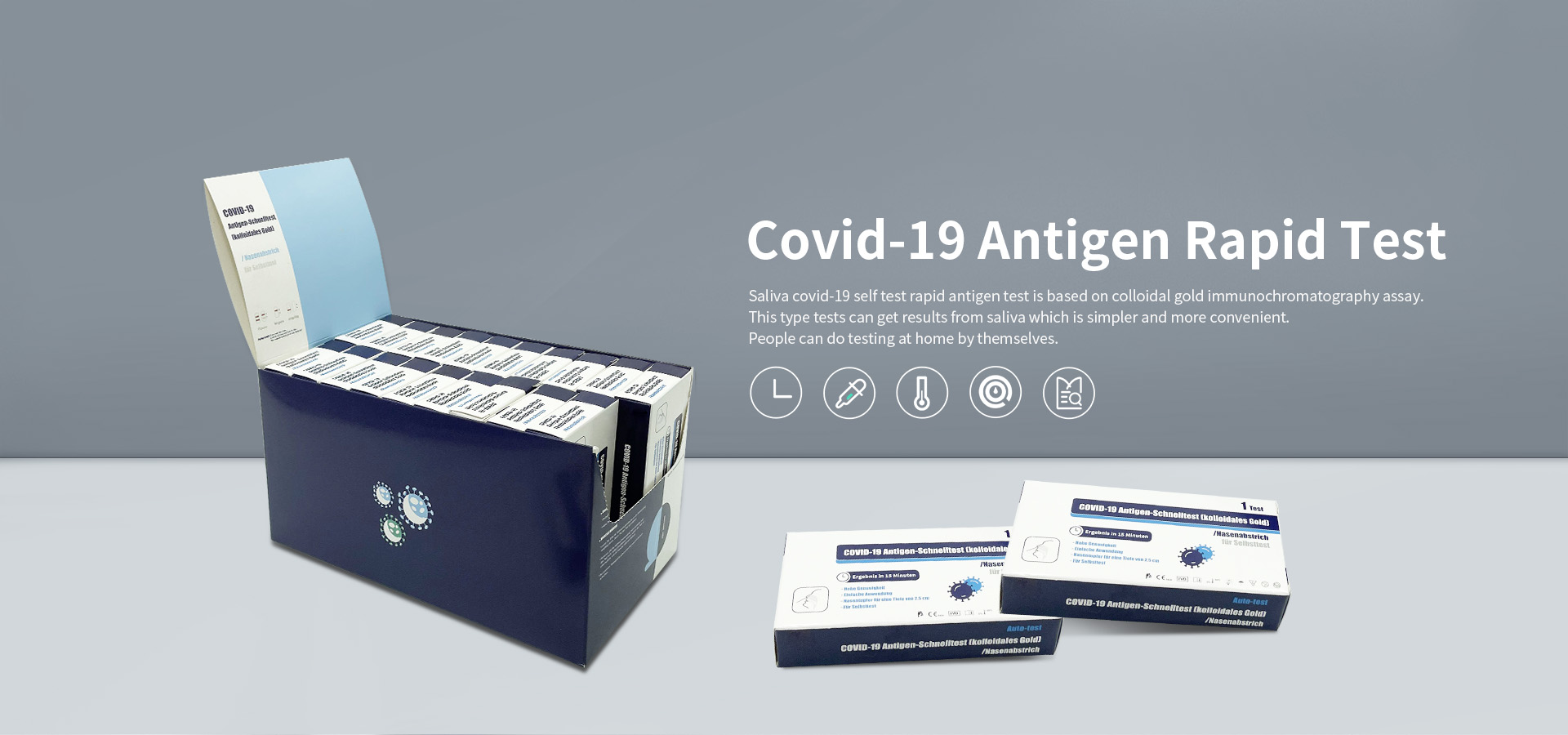
How to detect COVID-19 is a very important subject, and with continuous research and development and progress, there have been breakthroughs in this area. From clinical testing to self-testing is a great advancement. Our company manufactures face mask, simple operation covid-19 self test rapid antigen test, covid-19 self test rapid antigen test.
October 2020, COVID-19 Antigen test received CE certificates and export whitelist from EU.
December 2020, FDA approved the first home kit for COVID-19 antigen testing.
March 2021, German BFARM approved self-testing reagents for COVID-19 antigen.
April 2021, France, Netherlands, Denmark, Austria, Belgium, and other countries approved the COVID-19 self-testing reagent.
June 2021, COVID-19 antigen self-test kit received CE certificates from EU.
March 2022, Chinese National Medical Products Administration approved the list of the COVID-19 Antigen Self Test.